Research and development center
Drug Screening Research and Development Center
Drug Screening Research and Development Center
Drug screening and research and development center has been committed to technology research, chiral synthesis and separation, and all kinds of controlled release technology research and industrialization of research, have many years of research, tracking progress abroad, successfully developed a number of three kinds of new chemical API, and achieved a lot slow controlled release and other preparations of the new drug certificate and production approval, has the first-class national new drug research and the ability of quality standards, quality first in a series of dozens of agents and the quality of scientific research subject, accumulated a wealth of experience.
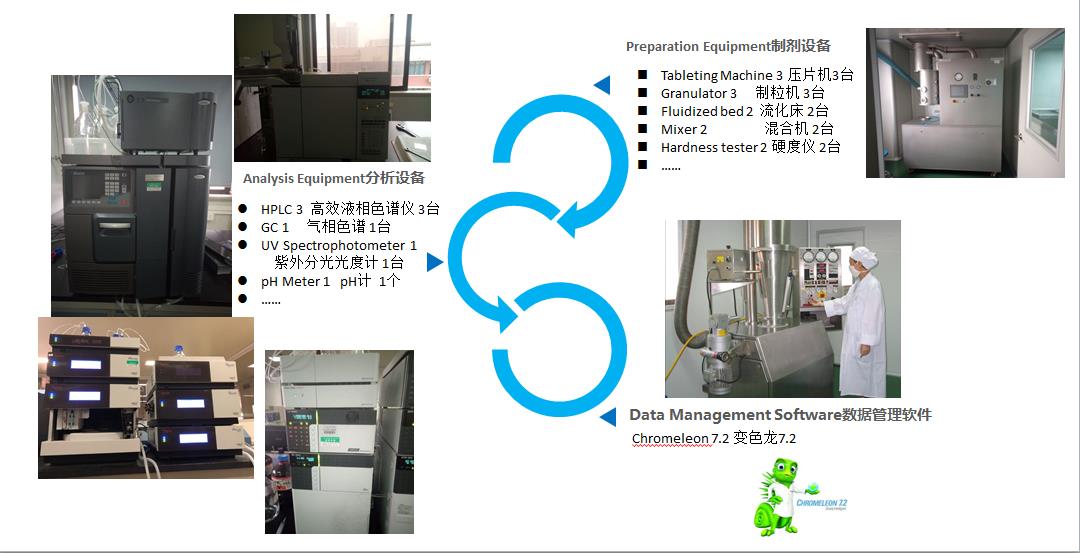
With an area of 1400m2, the center has a pharmaceutical preparation pilot test workshop, quality research laboratory and drug stability research laboratory designed in accordance with GMP requirements. Equipped with tablet, capsule and soft paste and other conventional preparation equipment, including the key equipment for the development of controlled and sustained release pellets -- fluidized bed (manufactured by Glatt in Germany), extruder-rotary machine (manufactured by Fuji-Paudal in Japan), can carry out preparation of various dosage forms and pilot scale and other process research; Equipped with a variety of precision and conventional detection equipment, including high-performance liquid chromatograph, gas chromatograph, ultraviolet spectrophotometer, dissolution determination instrument and other advanced analytical and testing instruments and physical and chemical testing equipment, can meet different levels of drug quality research. Meanwhile, the Chromeleon® chameleon software is equipped, and its data management conforms to GMP, 21CFR Part11 and other specifications. It can undertake chemical drug consistency evaluation, chemical drug preparation, quality standard research work (solid preparation, liquid preparation consistency evaluation research) and so on.


The center now has 16 researchers. Among them, there are 4 senior engineers, 4 engineers, 2 doctors and 6 masters. The center has a research team with doctor's degree as subject leader, doctor's degree as core backbone and professional technical background. The core team members have participated in the development of a number of new Chinese and western medicines and generic drugs, familiar with pharmaceutical preparation technology, analysis technology, understand the international development trend of pharmaceutical preparation, and familiar with drug policies. All team members have professional knowledge background and have received systematic quality management system training, and have the knowledge structure, work experience and business ability required to complete generic drugs and new drug research and development.
The center has been committed to the development of new drugs and generics, and the successful production of several research and development varieties has brought considerable economic benefits to manufacturers. He has won the national science conference award, the third prize of outstanding scientific and technological achievements of the state administration of medicine, the high quality product award of guangdong province, the first prize of scientific and technological achievements of guangdong medical system, the first prize of guangzhou scientific and technological achievements, the second prize of guangzhou new products, the second prize of guangzhou promotion and application achievements and other awards.
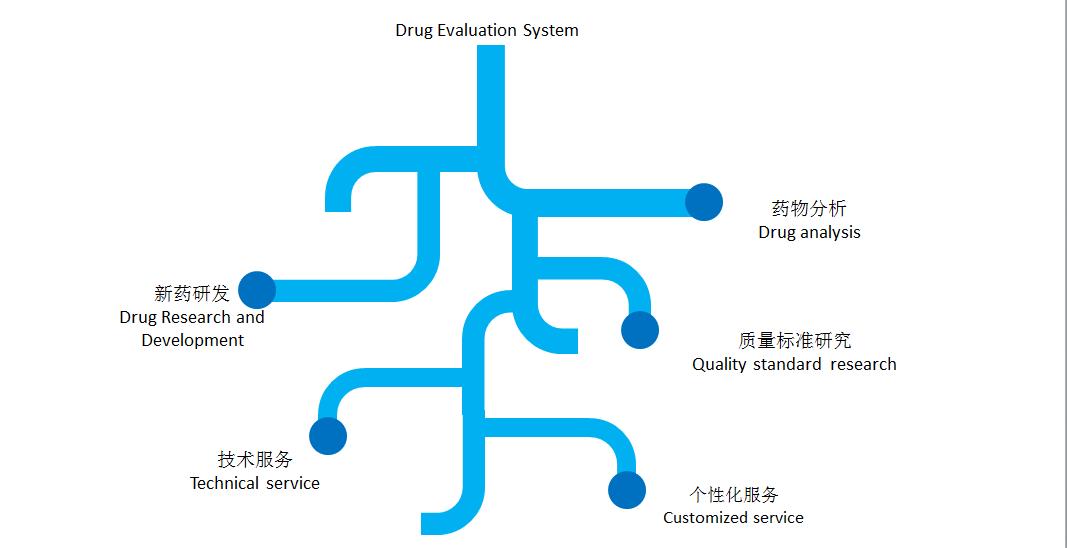
1. New drug development
It includes research and development, pilot test and industrial research of slow and controlled release preparation, oral preparation, cavity preparation, external application preparation and injection
2. Drug analysis and quality standard study
Study on quality standard of new drugs of traditional Chinese medicine and chemical drugs, improvement of original quality standard, study on quality of chiral drugs, determination of residual solvent
3. Technical services
Research and development of technologies to improve bioavailability, solubility and stability; Research and development of technology to improve dissolution (release) degree of preparation, tablet compressibility, technology industrialization transformation
4. Personalized service
According to the existing resources and product requirements of the enterprise, provide professional project planning and technical solutions, such as: project research and approval and application for government support, research and development of foreign new products to complete product chain, secondary development of existing varieties, and improve product quality